28 Mar NEW DIGITAL X-RAY DEVICE
“VISION U” BY VISARIS
VISION U AT THE INSTITUTE FOR ORTHOPAEDIC SURGERY „BANJICA“ IN BELGRADE
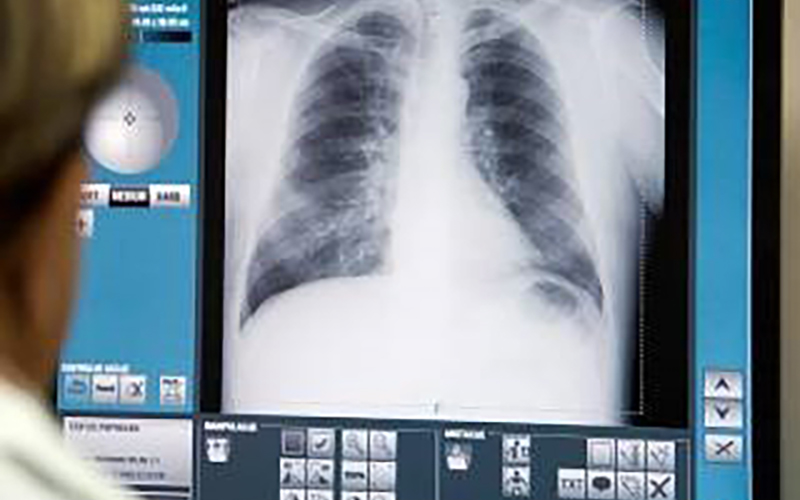
BELGRADE, 28 March 2011. Serbian company Visaris, the only manufacturer of modern digital X-ray machines in Southeast Europe, installed its latest digital X-ray machine called Vision U at the Institute for Orthopaedic Surgery “Banjica” in Belgrade. The machine was officially put in operation by Professor Doctor Slobodan Slavkovic, Director of the Institute, in the presence of numerous high-ranking officials.
Visaris donated and Visaris Diagon medical imaging workstation worth EUR 4.500 to the Institute.
Vision U is the latest generation of universal, motorized digital radiography (DR) systems for a wide range of general and specialist diagnostic imaging applications. Programmable auto-positioning of X-ray tube and large (43×43 cm) digital flat panel detector enable efficient and flexible imaging of all anatomies of both walking and immobile patients. Direct digital acquisition with optimally adjusted, anatomy specific imaging and image processing protocols produce clear visualization of all imaged anatomies. Single system console seamlessly controls all active system components meaning unparalleled user-friendly ergonomy and high workflow automation.

Visaris was founded in 2003 in Belgrade combining know-how and enthusiasm of young people who decided to apply their international experience and education in Serbia developing high-tech solutions in medical diagnostics. The company is unique not only in Serbia but also in the Southeast Europe region owing to its development program, design and solutions in the field of digital radiography and medical imaging. The company currently employs 34 people (average age is 32) and intends to hire another 30 in the upcoming period. Since 2006, 13 digital X-ray devices and 6 systems for digital image acquisition were installed in Serbia. Visaris’ products are also present in Czech Republic, Germany and South Africa.
Quality Policy
Visaris’ business fully complies with quality management systems and standards ISO9001 (General Quality Management Systems) and ISO13485 (Quality Management Systems for Medical Devices). The group of Visaris digital imaging systems for diagnostic X-ray technology complies with the EU Medical Device Directive 93/42/EEC and is eligible for the CE marking according to applied safety standards. Visaris’ devices are registered with the Medicines and Medical Devices Agency of Serbia (ALIMS) and have marketing authorization on the territory of the Republic of Serbia.