Highest safety standards
Visaris is fully committed to providing its customers with Digital Radiology products and services of consistently highest order of quality, safety and reliability.
In all our activities, from design and development to system production, implementation and support Visaris implements, maintains and continually improves relevant Quality Assurance systems that ensure highest product quality and safety.
Visaris’ quality system is ISO9001 (General quality management systems) and ISO13485 (Quality systems for medical device manufacturers) certified since 2005.
Visaris DR products conform to the highest safety standards as specified in the relevant international safety directives (MDD-93/42/EEC) and IEC standards such as IEC60601. Our Avanse range of acquisition systems is MDD (CE) approved.
Quality Policy
Visaris commitment to highest product safety and quality standards is embodied in our quality policy principles:
- All Visaris activities are dedicated to the needs and expectations of every customer and user through design and implementation of solutions that consider their specific needs
- It is our mission to strive to continually improve and advance all our products and services and offer these to our customers
- Visaris is fully committed to application of all internationally accepted safety standards to all its products and services
- Visaris implements and continually improves an integrated Quality Management System in all of its activities
- Visaris applies all of its expertise and technology in an ethical and open manner including information transfer, non-disclosure of customers’ sensitive information and above all patients’ privacy.
MDD 93/42/EC – CE MARK
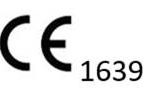
Visaris Avanse digital acquisition systems are MDD 93/42/EEC approved and bear the European safety standard CE mark. Read more: EC Certificate MDD
VISARIS QA MARK

All Visaris products are tested to the highest safety and performance standards and bear the mark of Visaris Quality Assurance.
ISO 9001 QMS

Visaris maintains an integrated Quality Management System according to ISO 9001:2015 international standard. Read more: Certificate ISO 9001_2015
ISO 13485 QMS

Visaris’ medical devices QMS is certified according to ISO 13485:2016 international standard for medical device manufacturers. Read more: Certificate ISO 13485_2016
ISO 14001 EMS

Visaris maintains an integrated Environmental Management System according to ISO 14001:2015 international standard. Read more: Certificate ISO 14001_2015
ISO 27001 ISMS

Visaris maintains an integrated Information security Management System according to ISO 27001:2022 international standard. Read more: Certificate ISO 27001_2022
ISO 45001 OHS
Visaris maintains an integrated Health and Safety Management System according to ISO 45001_2018 international standard. Read more: Certificate ISO 45001_2018
PRODUCT SUPPORT
Dedicated user-focused support system
Total commitment to empowering our partners to improve their performance and success levels through a reliable support, constant improvement and customization of all our products and services.
Visaris priorities when it comes to support are rapid response, availability, customization and product quality to ensure customers’ satisfaction in every situation.
Our support teams are made up of experienced engineers with years of experience in providing an immediate support worldwide.
Out total commitment to support and service of the highest quality for all our Visaris Digital Radiology customers is ensured through:
- Guaranteed response times (including immediate response)
- Timely and targeted intervention
- Quality Management Systems for constant service and support improvements
- Readiness, reliability, and effectiveness
- 24×7, 365 days a year
- Online tracking, reporting and feedback
REQUEST SUPPORT
send e-mail to our support
CALL VISARIS DR SUPPORT
+381 11 2017655